High-performance nitrous oxide decomposition catalyst...Possibility in usage on various metal catalyst studies
As the United Nations Convention on Climate Change recently announced a high-intensity methane regulation plan to solve the global warming problem, solutions to the greenhouse gas reduction problem have become a global topic.
In general, methane(CH4) and carbon dioxide(CO2) are cited as greenhouse gases that affect global warming, and nitrous oxide(N2O) tends to be neglected. In fact, nitrous oxide is produced in a relatively small amount, but its global warming potential(GWP) is more than 300 times that of methane and carbon dioxide, and it must be managed for more than 100 years to naturally decompose.
In this situation, Professor Kim Kyung-hak(photo·31) from Hanyang University Department of Chemical Engineering recently succeeded in developing a highly efficient and stable nitrous electrocatalyst for N2O reduction through joint research with Seoul National University Professor Kim Jae-jung, Professor Hyun Taek-hwan, and POSTECH Professor Han Jung-woo research teams. Commercialization of the substance is expected to greatly contribute to solving the global warming problem.
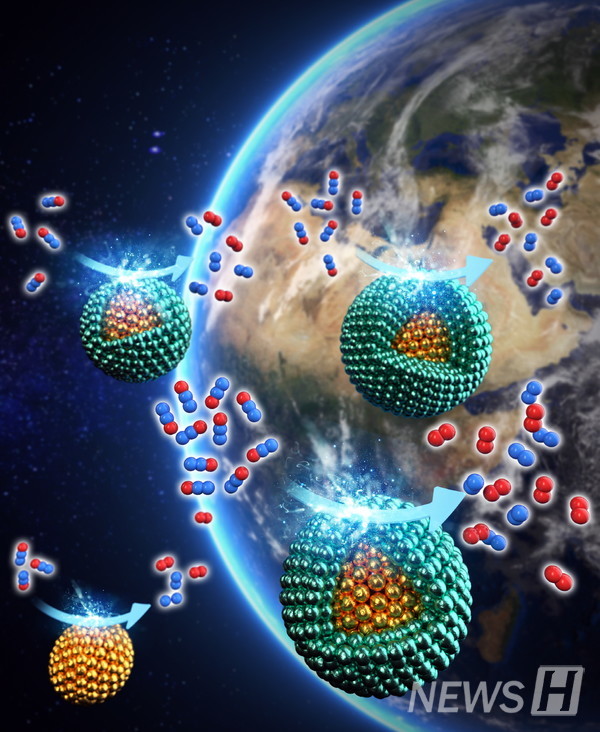
Professor Kim Kyung-hak focused on the point that when N2O meets a palladium(Pd), it decomposes into nitrogen and oxygen and that the performance of palladium is greatly improved when the tensile strain is applied to the surface of the palladium. However, there were problems with previous technology in that additional facilities and operating costs were required to apply physical forces or maintain changes in environmental conditions to efficiently apply tensile force to palladium.
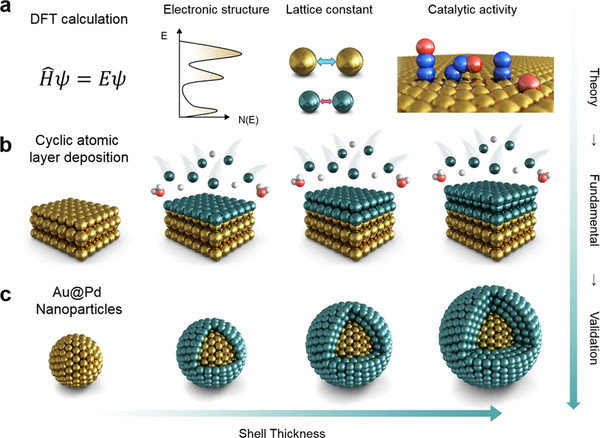
To solve this problem, Professor Kim used gold(Au) with a larger atomic radius than palladium. Professor Kim devised a "core-shell structure", making nanoparticles out of gold and overlayering Pd atomic layers on the surface of nanoparticles. He also developed a new catalyst with increased catalytic activity and stability, by optimizing Pd shell thickness and applying a tensile force to palladium without consuming external energy.
The nanocatalyst designed in this way showed a significant increase in nitrous oxide decomposition capacity compared to the existing catalyst, and even after 1000 performance tests, it showed a 30% improvement in stability compared to the previous one. This shows strengths in both activity and stability, which are key indicators of catalytic performance.
This study is significant in that it reduced the time and cost required for experiments and increased research efficiency by performing catalyst materials screening and identifying mechanisms of catalyst phenomena through computational and chemical methodologies that have recently drawn attention.
Professor Kim Kyung-hak, who designed and led the research, said, "The results of this study can be directly applied to solving the global warming problem, and it is expected that the theory-experiment-demonstration research methodology used in this study can be applied in designing various catalysts based on metal nanoparticles."
Meanwhile, the study was conducted with the help of the Korea Research Foundation's junior researcher support project and the IBS Research Group. Also, it was published as the cover paper in the December issue of 「ACS Catalysis」(Systematic Approach to Design of Highly Efficient Core-Shell Electrocatalysts for N2O Reduction) a world-renowned catalyst field.

■ Paper title : Systematic Approach to Design of Highly Efficient Core-Shell Electrocatalysts for N2O Reduction
■ Author Information: Professor Kim Kyung-hak(lead author, Hanyang University), Byun Jin-wook(lead author, Seoul National University), Kim Hyun-joong(lead author, Seoul National University), Dr. Lee Kuk-seung(Pohang Accelerator Center), doctoral program Lee Hyun-seok(Seoul National University), doctoral program Kim Ji-heon(Seoul National University), Professor Hyun Taek-hwan(corresponding author, Seoul National University), Professor Kim Jae-jung(corresponding author, Seoul National University), Professor Han Jeong-woo(corresponding author, POSTECH).
키워드
 '한양위키' 키워드 보기
#SDG13
'한양위키' 키워드 보기
#SDG13

